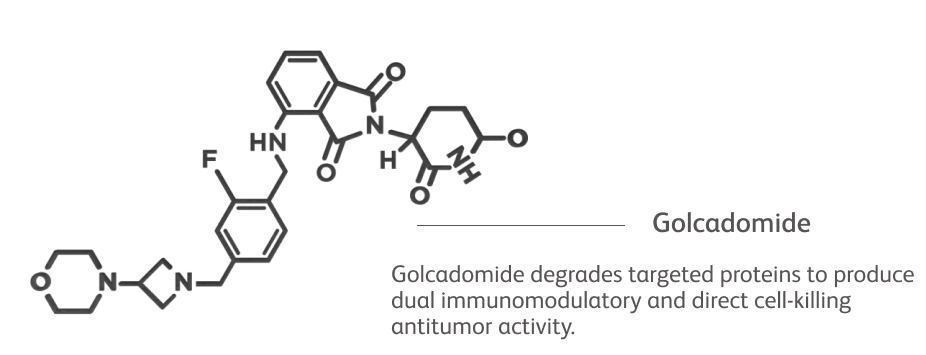
How Golcadomide Works in NHL

How Novel CELMoD Agents Work*
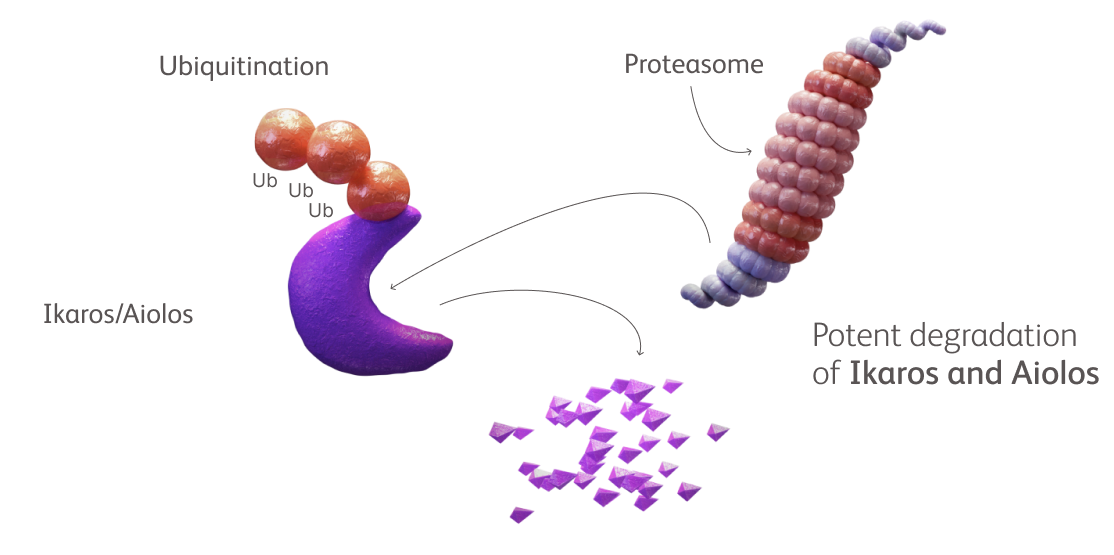
Novel CELMoD agents, also called next-generation IMiD® agents, are purposefully designed to enhance targeted protein degradation vs classic IMiD agents in multiple myeloma.

cereblon is co-opted
Novel CELMoD agents iberdomide and mezigdomide are biochemically distinct molecules that bind to cereblon with higher specificity and affinity than classic IMiD agents.1-5,*
Enhancing targeted protein degradation
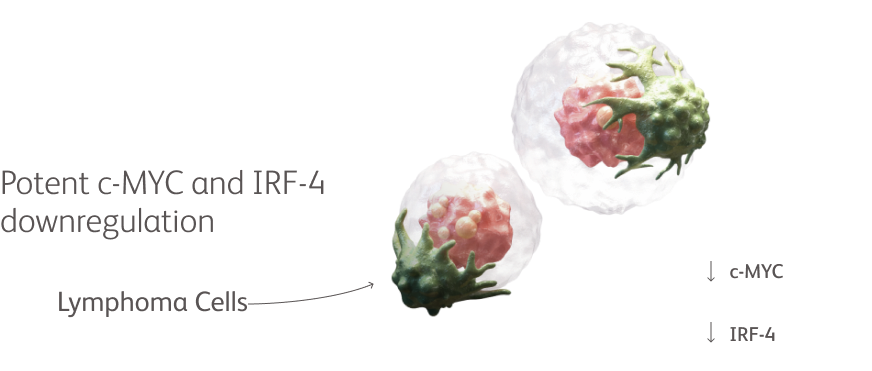
Co-opting cereblon induces deeper and more rapid targeted degradation of key proteins important for myeloma cell growth and survival compared with classic IMiD agents.1-5,*

Leading to potent anti-myeloma effects
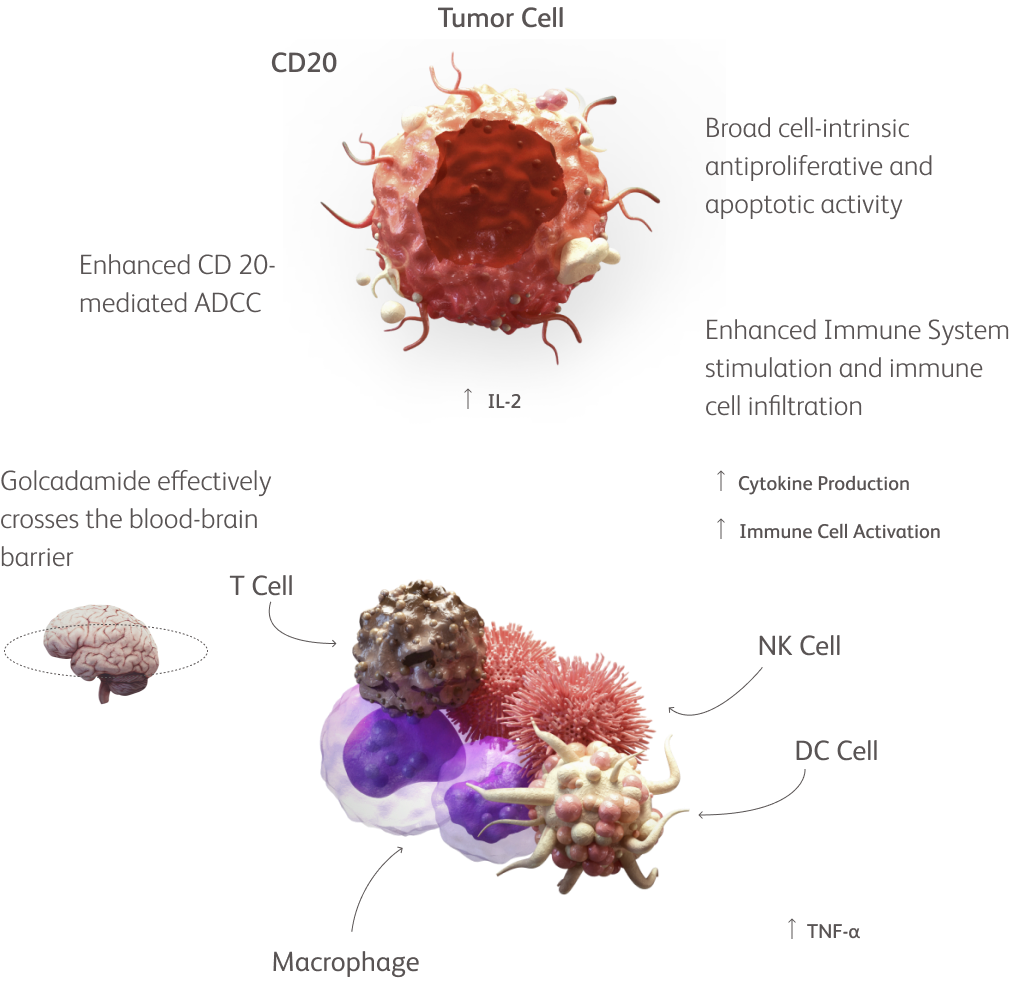
By enhancing targeted protein degradation with high efficiency, novel CELMoD agents can directly kill myeloma cells and stimulate the immune system.1-5
1. Kercher T, et al. Oral presentation at ACS; August 22–26, 2021; Atlanta, GA, USA. Presentation 3584532; 2. Carrancio S, et al. Poster presentation at ASH Annual Meeting; December 11–14, 2021; Atlanta, GA, USA. Poster 1200. Abstract 605; 3. Lopez-Girona A, et al. Poster presentation at ICML; June 18–22, 2021; Virtual. Abstract 232; 4. JanardhananP, et al. Poster presentation at the AACR Annual Meeting; April 5–10, 2024; San Diego, CA. Presentation 3305.
Novel CELMoD agents, as observed in preclinical studies, are:
-
MoDeled after classic IMiD agents but have chemical structures purposefully designed to enhance binding to cereblon and targeted protein degradation1-3
-
MoDified for potent killing of myeloma cells and strong immune stimulation (even in a T-cell–exhausted setting) via oral administration1-5
-
MoDernized to provide enhanced synergistic anti-myeloma in vitro, including in IMiD-resistant settings. Combination regimens are now being investigated in phase 3 clinical trials1-9
*Based on preclinical in vitro studies, the mechanism of action statements are not meant to imply clinical outcomes.
Clinical Trials6-9
Phase 3 clinical trials of novel CELMoD agents iberdomide and mezigdomide are currently open for enrollment.
-
Trials include patients being treated in 1L maintenance, post-SCT, and in early/late RRMM settings
-
Novel CELMoD agents are being studied as monotherapy and in combination with other proven drug classes such as PIs and anti-CD38 mAbs
These trials are investigating new therapeutic options to improve the standard of care across the treatment spectrum for patients with multiple myeloma.
These are investigational products being studied for the treatment of multiple myeloma and have not been approved for use in any country.
Find the right golcadomide clinical trial for your patient with lymphoma
Confirm EligibilityFind a golcadomide clinical trial by location
CELMoD=cereblon E3 ligase modulator: D=Darzalex® (daratumumab); d=dexamethasone; Iber=iberdomide; K=Kyprolis® (carfilzomib); mAb=monoclonal antibody; Mezi=mezigdomide; NDMM=newly diagnosed multiple myeloma; PI=proteasome inhibitor; Pom=POMALYST® (pomalidomide); R=REVLIMID® (lenalidomide); RRMM=relapsed/refractory multiple myeloma; SCT=stem cell transplant; V=Velcade® (bortezomib).
References: 1. Bjorklund CC, Kang J, Amatangelo M, et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia. 2020;34(4):1197-1201. doi:10.1038/s41375-019-0620-8 2. Matyskiela ME, Zhang W, Man H-W, et al. A cereblon modulator (CC-220) with improved degradation of Ikaros and Aiolos. J Med Chem. 2018;61(2):535-542. doi:10.1021/acs.jmedchem.6b01921 3. Hansen JD, Correa M, Nagy MA, et al. Discovery of CRBN E3 ligase modulator CC-92480 for the treatment of relapsed and refractory multiple myeloma. J Med Chem. 2020;63(13):6648-6676. doi:10.1021/acs.jmedchem.9b01928 4. Lonial S, Amatangelo M, Popat R, et al. Preclinical, translational, and clinical evidence of a differentiated profile for the novel CELMoD, iberdomide (CC-220). Poster presented at: 61st Annual Meeting of the American Society of Hematology (ASH); December 7-10, 2019; Orlando, FL. 5. Lonial S, Popat R, Hulin C, et al. Iberdomide in combination with dexamethasone in patients with relapsed/refractory multiple myeloma: results from the dose-expansion phase of the CC-220-MM-001 trial. Presented at: 63rd Annual Meeting of the American Society of Hematology (ASH); December 10-14, 2021; Atlanta, GA. 6. A phase 3, two-stage randomized, multicenter, open-label study comparing iberdomide, daratumumab and dexamethasone (IberDd) versus daratumumab, bortezomib, and dexamethasone (DVd) in subjects with relapsed or refractory multiple myeloma (RRMM). ClinicalTrials.gov identifier: NCT04975997l. Updated October 17, 2023. Accessed October 31, 2023. https://www.clinicaltrials.gov/study/NCT04975997 7. A phase 3, two-stage, randomized, multi-center, controlled, open-label study comparing iberdomide maintenance to lenalidomide maintenance therapy after autologous stem cell transplant (ASCT) in participants with newly diagnosed multiple myeloma (NDMM) (EXCALIBER-MAINTENANCE). ClinicalTrials.gov identifier: NCT05827016. Updated October 26, 2023. Accessed October 31, 2023. https://www.clinicaltrials.gov/study/NCT05827016 8. A phase 3, two-stage, randomized, multicenter, open-label study comparing mezigdomide (CC-92480), bortezomib and dexamethasone (MEZIVd) versus pomalidomide, bortezomib and dexamethasone (PVd) in subjects with relapsed or refractory multiple myeloma (RRMM): SUCCESSOR-1. ClinicalTrials.gov identifier: NCT05519085. Updated October 27, 2023. Accessed October 31, 2023. https://www.clinicaltrials.gov/study/NCT05519085 9. A phase 3, two-stage, randomized, multicenter, open-label study comparing mezigdomide (CC-94280/BMS-986348), carfilzomib, and dexamethasone (MeziKD) versus carfilzomib and dexamethasone (Kd) in participants with relapsed or refractory multiple myeloma (RRMM): SUCCESSOR-2. ClinicalTrials.gov identifier: NCT05552976. Updated October 31, 2023. Accessed October 31, 2023. https://www.clinicaltrials.gov/study/NCT05552976 10. Shi Q, Chen L. Cereblon: a protein crucial to the multiple functions of immunomodulatory drugs as well as cell metabolism and disease generation. J Immunol Res. 2017;2017:9130608. doi:10.1155/2017/9130608